Chiral α-branched allylic amines are widely employed in the chemical industry and as intermediates in a large number of natural product syntheses, since their amino and olefin functionalities allow for a range of chemical manipulations. Among the established routes to chiral allylic amines is the [3,3]-rearrangement of allylic imidates. However, these substrates are sensitive towards hydrolysis, which hampers their long-term storage, and their preparation is tedious.
René Peters, University of Stuttgart, Germany, and colleagues have developed an alternate route to chiral allylic amines by catalytic asymmetric rearrangement of allylic carbamates, a similar precursor to allylic imidates, albeit with higher stability and easier preparation. The rearrangement product also spontaneously decarboxylates negating the need for an extra deprotection step.
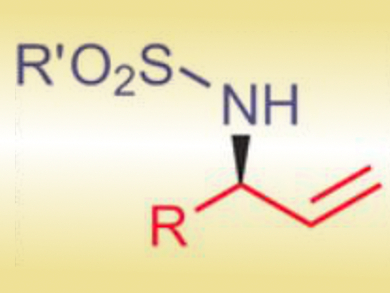
Through cooperative catalysis by PdII and a tertiary amine, the researchers achieved a one-pot domino conversion of achiral allylic alcohols into tosyl-protected chiral allylic amines (pictured). Notably, the reaction does not require catalyst activation or an inert atmosphere.
- Dual Palladium(II)/Tertiary Amine Catalysis for Asymmetric Regioselective Rearrangements of Allylic Carbamates,
Johannes Moritz Bauer, Wolfgang Frey, René Peters,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201600138