Photoelectrochemical water splitting via solar energy is been widely regarded as one of the most promising routes for hydrogen production. The first step of water splitting, the oxygen evolution reaction (OER) is a thermodynamically uphill reaction that involves a four-electron oxidation process of water molecules. It leads to the sluggish water oxidation kinetics on many promising semiconductors, such as Fe2O3.
The use of cocatalysts has been an established approach to accelerate the sluggish water oxidation kinetics for water splitting. While many single cocatalyst configurations have been well documented, cocatalytic systems that consist of multiple components with different functions may allow for better performance.
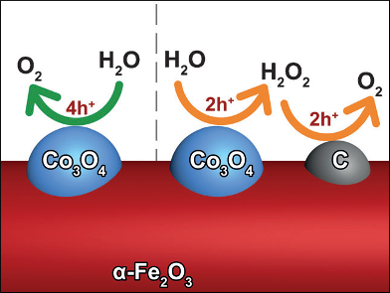
Jinlong Gong, Tianjin University, China, and colleagues have synthesized a cocatalyst composed of carbon nanodots adjacent to Co3O4, which has a synergistic catalytic effect that promotes the OER on Fe2O3. When the two cocatalysts are loaded simultaneously, a kinetically favored two-step-two-electron reaction pathway is established. Initially, the water molecule is oxidized to H2O2 at the Co3O4. The produced H2O2 is further oxidized to O2 on carbon nanodots.
The kinetically favored reaction pathway and the enhanced surface reaction rate result in an improved OER activity of the modified Fe2O3 photoanodes, exhibiting a nearly doubled photocurrent density compared to bare Fe2O3. The discovery of this synergistic effect between different cocatalysts provides insights into the rational design of effective cocatalytic systems with multiple functional components, which should also be applicable to other energy conversion applications.
- Synergistic Cocatalytic Effect of Carbon Nanodots and Co3O4 Nanoclusters for the Photoelectrochemical Water Oxidation on Hematite,
Peng Zhang, Tuo Wang, Xiaoxia Chang, Lei Zhang, Jinlong Gong,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201600918