Light-Controlled Reaction Manifold
An international collaboration between chemists from Germany, Australia, and Belgium has led to an efficient reaction manifold that follows one of two paths depending on whether the system is illuminated or kept in the dark.
Christopher Barner-Kowollik, Karlsruhe Institute of Technology, Germany, and Queensland University of Technology (QUT), Brisbane, Australia, together with James P. Blinco at QUT and colleagues have added a new twist to photochemistry. Their thermally induced ligation reaction can be controlled by light via two competing reaction channels.
Diels-Alder Reaction vs. Imine Formation
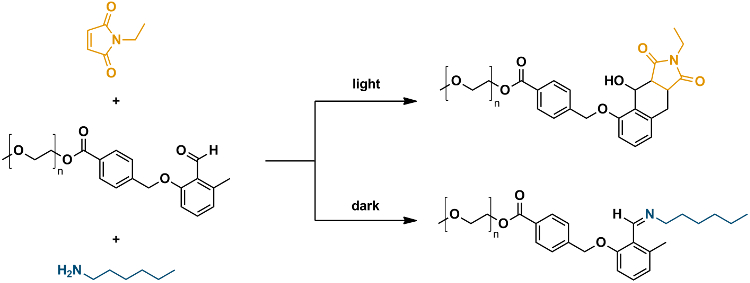
The system comprises an o-quinodimethane species, a photocaged diene which reacts with suitable enes in a conventional Diels-Alder reaction in the presence of light, but undergoes a transformation into imine products with amines when there is no light (pictured below). The team demonstrated the principle by carrying out the transformations on the ends of macromolecular chains, and monitored the reaction by using high-resolution mass spectrometry and watching for selective block copolymer formation.
“The chemical selectivity of the manifold is controlled by the amount of ene present in the reaction and can be adjusted from 100 % imine formation (0 % photo product) to 5 % imine formation (95 % photo product),” the team explains.
Controlling One-Pot Reactions
Orthogonal reactions have become a popular feature of chemical synthesis in recent years not least since the advent of “click” chemistry. A complicated target molecule is constructed in a single reaction vessel, one pot, through independent processes that bring together different starting materials to generate intermediates that then react in the same vessel to give the final product.
However, one problem – that those hoping to exploit the inherent simplicity of a one-pot scheme – is that thermal control is not all that it’s cracked up to be and spatial and temporal control are not feasible. Laser pulses can be used to add a dimension of temporal control in photochemical curing. Spatial control using light has been employed before. Yet a combined thermal-light manifold as used in this study would in the scientists’ opinion give chemists much greater control of all factors and open up multiple ways to convert ubiquitous off-the-shelf starting materials into sophisticated end products.
Switching Reactivity On and Off
It was well known that o-quinodimethanes, which are the photoenol product of o-methyl benzaldehydes, undergo a phototriggered [4 + 2]-cycloaddition with electron-poor enes such as maleimides, dithioesters, and diphenylcarbenes. The team points out that the photoenolization-cycloaddition ligation is classified as a light-induced click reaction and some of the researchers have previously exploited this in block copolymer synthesis, the production of cyclic polymers, nanoparticle modification, surface patterning, and 3D micro scaffold construction. The team’s light-controlled manifold takes this a step further and allows them to control the pathway at different stages simply by switching the light on or off.
The researchers explain the nature of this chemical switching: “The system rests on the ability of an aryl aldehyde (prior to photoenolization) reacting with amines to yield a stable imine. However, when exposed to light activation the aryl aldehyde is readily transformed into a diene (o-quinodimethane or photoenol), which undergoes a Diels-Alder reaction with a maleimide and in its diene form is no longer able to undergo a thermal reaction with amines.”
Michelle Coote, Australian National University, Canberra, told ChemViews Magazine, “This is very clever chemistry that exploits the photoenolisation of aryl aldehydes to use light to switch between imine formation and [4 + 2]-cycloaddition. Because the photoreactions can be switched on and off so quickly, this is a very convenient tool for controlling the sequence of reactions in organic synthesis.”
She adds that it could have immediate important applications in the synthesis of well-defined block copolymers and dendrimers. “One could imagine this chemistry being harnessed for sequence control applications in polymerization,” she suggests. “Considering [4 + 2]-cycloaddition reactions can themselves undergo chemoselective switching with heat, pH and potentially electric fields, the addition of light to this tool box adds an exciting additional dimension to our ability to control chemical reactions.”
- A Light-Activated Reaction Manifold,
Kai Hiltebrandt, Katharina Elies, Dagmar R. D’hooge, James P. Blinco, Christopher Barner-Kowollik,
J. Am. Chem. Soc. 2016.
DOI: 10.1021/jacs.6b01805