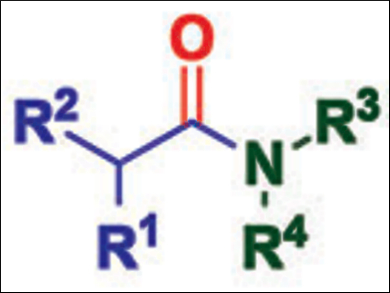
A combination of photoredox catalysis and aminocarbonylation has been used for the first time to prepare compounds that cannot be made using conventional metal catalysis.
Luke R. Odell and colleagues, Uppsala University, Sweden, have developed a fac-Ir(ppy)3-mediated radical aminocarbonylation of unactivated alkyl iodides (see below). The initial generation of alkyl radicals through a radical reductive dehalogenation is followed by an aminocarbonylation that affords a wide range of alkyl amides in moderate to excellent yields.
.png)
In comparison with the existing alternatives, this method avoids the use of CO gas at high pressure and therefore the hazards associated with its handling. Various CO sources were also tested and several alternatives were identified, although Co(CO)6 proved to work best.
- Mild and Low-Pressure fac-Ir(ppy)3-Mediated Radical Aminocarbonylation of Unactivated Alkyl Iodides through Visible-Light Photoredox Catalysis,
Shiao Y. Chow, Marc Y. Stevens, Linda Åkerbladh, Sara Bergman, Luke R. Odell,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201601694