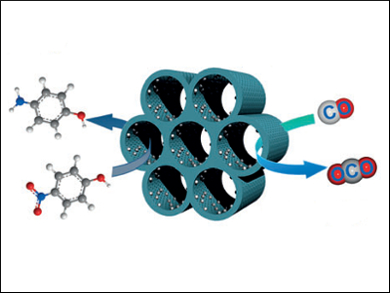
The use of platinum in catalysis is very common due to its typically high reactive reliability. To increase catalyst effectiveness and to reduce costs, using catalyst nanoparticles can be highly advantageous. They have greater overall surface areas and could allow for much lower catalytic loadings. However, they can clump together, reducing surface area and hence, efficiency, and they can be hard to recover for reuse when a reaction is finished.
Zhongmin Su, Northeast Normal University and Jilin University, both Changchun, China, and colleagues have used a mesoporous support, which consists of silica and a phenolic resin, to try and overcome these problems. Platinum nanoparticles were dispersed on this support to avoid aggregation. The catalyst was prepared by reacting the ordered mesoporous polymer-silica support with Pt2+ ions, which were converted to Pt nanoparticles in situ. The polymer was then removed by calcination in air.
The resulting platinum-loaded (1 wt%) silica was tested for its catalytic activity. In the gaseous oxidation of CO, high conversions were achieved at high temperatures (100 % at 220 ˚C). The catalytic activity remained excellent over ten cycles, showing a high thermal stability. The catalyst was also tested in the liquid-phase reduction of nitrophenol to aminophenol in the presence of NaBH4. Here, it showed remarkably quick reaction times as well as a high activity after multiple uses.
- Facile Fabrication of Well-Dispersed Pt Nanoparticles in Mesoporous Silica with Large Open Spaces and Their Catalytic Applications,
Xianchun Liu, Dashu Chen, Lin Chen, Renxi Jin, Shuangxi Xing, Hongzhu Xing, Yan Xing, Zhongmin Su,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201600894