Producing Talc Within Seconds
It takes millions of years until the natural clay mineral talc is produced hydrothermally under bedrock, but artificial synthesis can make this process a lot shorter. Synthetic talc has many advantages such as high purity and tunable properties. In the journal Angewandte Chemie, French scientists introduce a laboratory synthesis of talc, which needs only seconds and produces nanocrystals with unique properties useful in many applications.
Talc is a nontoxic clay mineral, which is widely used in various industries. In chemical terms, it is a hydrated magnesium silicate, the deposits of which are mined worldwide. However, the mined talc faces challenges mainly concerning homogeneity and purity.
Supercritical Hydrothermal Flow Synthesis
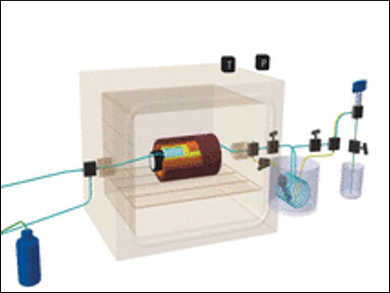
Two research groups under the direction of Cyril Aymonier and François Martin in Bordeaux and Toulouse, France, have developed a method to produce synthetic talc nanocrystals hydrothermally in supercritical water. In this state, water has properties neither of a liquid nor of a gas and offers unique conditions for hydrothermal synthesis.
“Water properties under supercritical conditions can be finely tuned from gas-like to liquid-like,” the researchers write. And while the natural process to produce talc, which is metamorphosis under bedrock, leads to micron-sized, rather impure and hydrophobic talc crystals, Aymonier and Martin’s “supercritical hydrothermal flow synthesis” produces pure nanocrystals within seconds.
New Opportunities for Industrial Applications
This technology also offers access to the unique characteristics of talc nanocrystalline structures. The scientists point out that nanominerals have long been overlooked in natural ore, although they can severely affect its properties. “Even in small proportion, nanominerals can induce defect of ore-grade material through their reactivity,” the authors write, highlighting the importance of studying the growth behavior and characteristics of the nanominerals.
The hydrothermally synthesized talc nanocrystals were also shown to present some remarkable features. In contrast to their micron-sized natural counterparts, they are hydrophilic and thus offer new opportunities for industrial applications. Therefore, further exploration as reinforcement, for paints, and in the paper, ceramics, and cosmetics industries is recommended.
- Fast-Geomimicking using Chemistry in Supercritical Water,
Angela Dumas, Marie Claverie, Cédric Slostowski, Guillaume Aubert, Cristel Careme, Christophe Le Roux, Pierre Micoud, François Martin, Cyril Aymonier,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201604096