The amine-catalyzed 1,4-reduction of α,β-unsaturated aldehydes provides chemists with a wide range of optically active molecules. A Hantzsch ester derivative usually performs the role of the reductant in such reactions and reduces the in situ-formed iminium species.
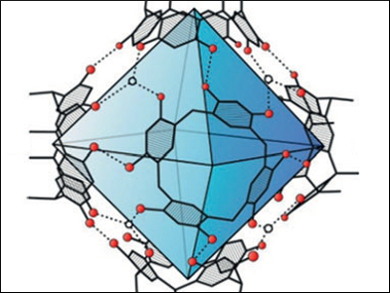
Konrad Tiefenbacher, University of Basel, Switzerland, and colleagues have performed such reactions inside a self-assembled container molecule (pictured). The container is formed by self-assembly from six resorcinarene units and eight water molecules. The formed iminium species is encapsulated reversibly inside the container and reacts within the closed environment. In general, higher enantioselectivities were obtained inside the container than in control experiments without the host structure. There are some parallels to natural imine reductases, in which NADPH functions as a cofactor and binds inside enzyme pockets.
- Iminium Catalysis inside a Self-Assembled Supramolecular Capsule: Modulation of Enantiomeric Excess,
Thomas M. Bräuer, Qi Zhang, Konrad Tiefenbacher,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201602382