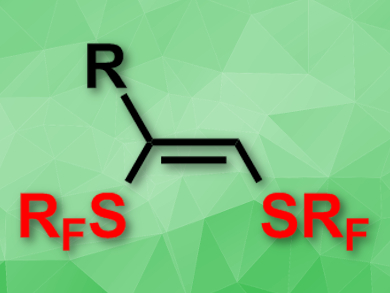
The incorporation of SCF3 units into organic compounds has recently attracted much attention due to properties such as increased lipophilicity caused by these units. The key to the introduction of SCF3 units is the choice of trifluoromethylthiolating reagent. Nucleophilic donors, such as AgSCF3 and CuSCF3, are most commonly used. Electrophilic reagents, such as F3CSCl and F3CSSCF3, are also known, though these are often inconvenient or toxic. Additionally, basic conditions or high temperatures have proved problematic for sensitive substrates.
Anis Tlili, Thierry Billard, and colleagues, University of Lyon, France, have developed a copper-catalyzed strategy for alkyne trifluoromethylthiolation that does not require activation with a base and can be performed at room temperature. By association of trifluoromethanesulfenamides with CuI and 2,2′-bipyridine, a wide range of alkyne substrates underwent mono- or bis-trifluoromethylthiolation with Z-selectivity (product pictured). Furthermore, the strategy could be extended to allow the introduction of higher perfluoroalkythiol groups, e.g., SCF2CF3 and S(CF2)2CF3.
- Copper-Catalyzed Perfluoroalkylthiolation of Alkynes with Perfluoroalkanesulfenamides,
Anis Tlili, Sébastien Alazet, Quentin Glenadel, Thierry Billard,
Chem. Eur. J. 2016, 22, 10230–10234.
DOI: 10.1002/chem.201601338