Rapid Determination of Erythrocyte Counts
Blood counts are routinely carried out before operations, in cases of infection, or when testing for a variety of diseases, such as anemia and leukemia. A key value in this test is the number of red blood cells (erythrocytes).
Scientists at the University of Oxford, UK, have developed a simple nano-electrochemical process for the rapid, precise determination of the erythrocyte count. As described in the journal Angewandte Chemie, the test also determines the activity of individual erythrocytes toward hydrogen peroxide.
Simple Electrochemical Measurements
The time-consuming process of counting blood cells under a microscope has been replaced by automated techniques. However, these still require complex instruments. It would be nice to have a method that could deliver equally precise results at the point of care, in a quick, simple and inexpensive way. The team led by Richard G. Compton has developed a new method to meet these requirements.
A sample of 5 µL of diluted blood is placed on a special biocompatible graphite electrode, and voltammograms are obtained. Pure buffer solution gives a slight electrochemical signal at the electrode caused by the reduction of the small amount of dissolved oxygen. Samples with blood give a correspondingly higher signal, because the red blood cells contain oxygen. The signal strength can be used to determine the erythrocyte concentration with great precision. The only equipment necessary is an electrode and a commercial electrochemical device.
Catalytically Active Red Blood Cells
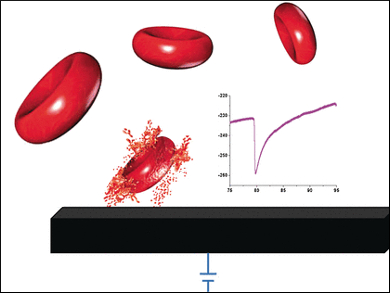
Addition of hydrogen peroxide strengthens the signal obtained because it is converted to oxygen and water by the enzymes glutathione peroxidase and catalase within the red blood cells. This process is used by the cells to neutralize toxic material. The scientists replaced the graphite electrode with a carbon microelectrode and recorded the current under constant voltage over time. This measurement resulted in spikes: rapid decrease in the current is followed by a slow return to the background signal. The frequency of the spikes increases with increasing erythrocyte concentration.
“Each individual spike can be ascribed to an individual red blood cell catalytic activity when colliding with the electrode,” explains Compton. “At the electrode, the local concentration of hydrogen peroxide is very high if the voltage is high enough. Cells that come very close to the electrode are attacked by the peroxide, torn open, and enzymes released that destroy the local peroxide. This causes the current to decrease rapidly. As the enzymes diffuse away, the current slowly returns to its original value.”
These measurements deliver complementary information, because both the number and peroxidase activity of individual erythrocytes can be determined. In certain diseases, this activity may change and can be monitored at the point of care.
- Electrochemical Red Blood Cell Counting: One at a Time,
Lior Sepunaru, Stanislav V. Sokolov, Jennifer Holter, Neil P. Young, Richard G. Compton,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201605310