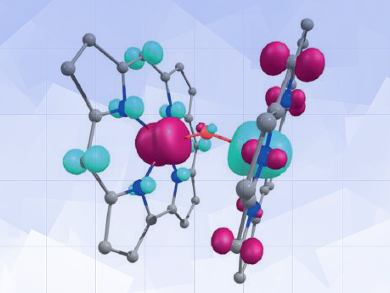
Iron(IV)-oxo species have long been studied by bioinorganic chemists for their unique reactivities. However, most synthetic species of this type are also highly unstable. In light of this, the stability of μ-oxo-diiron corroles, discovered in 1994, was surprising. For over 20 years, these species were thought to contain a pair of antiferromagnetically coupled low-spin iron(IV) centers.
Hugo Vazquez-Lima, Abhik Ghosh and colleagues, Arctic University of Norway, Tromsø, have performed a theoretical and experimental study which calls this into question. Several metallocorroles, including those with Cu, Fe, and Pt, show ligand non-innocence; that is, the corrole ligand is a corrole.2– radical rather than corrole3–. These ligands exhibit bond-length alternation in the crystal structure, which is also the case for μ-oxo-diiron corroles.
Detailed density functional theory (DFT) calculations on the μ-oxo-diiron corrole {Fe[TPC]}2O (TPC = triphenylcorrole) indicated that this species exists not, as previously believed, as an iron(IV)-oxo species, but as an assembly of four open-shell fragments, corrole·2–(↓) − FeIII(↑↑↑) − FeIII(↓↓↓) − corrole·2–(↑) (arrows indicate up and down spin, respectively). Charge delocalization with ligand non-innocence helps explain the surprising stability.
- Wolves in Sheep’s Clothing: μ-Oxo-Diiron Corroles Revisited,
Sumit Ganguly, Hugo Vazquez-Lima, Abhik Ghosh,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201601062