To gain an understanding of catalytic processes, stoichiometric reactivity studies are often performed. Oxidative addition (OA) is an important initial step in many organometallic catalysis reactions and is often used to synthesize key metal intermediates involved in the catalysis. Thus, it proves useful to first study OA by forming target metal complexes, because this can provide evidence for the mechanism of the corresponding catalytic reaction.
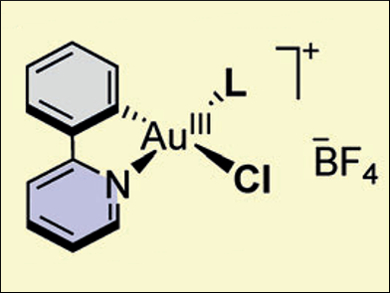
Frank Glorius and colleagues, Westfälische Wilhelms-Universität Münster, Germany, have performed a successful OA of aryldiazonium salts to gold(I) complexes under visible-light photoredox conditions. The researchers use a fast and operationally simple method to access a wide range of cationic (C,N)-cyclometalated gold(III) species (pictured). The photoredox reaction conditions use a ruthenium-based photocatalyst and green light-emitting diodes (LEDs) as the visible-light source.
Using this novel photoredox strategy, the researchers successfully prepared gold(III) species bearing a variety of phosphine and N-heterocyclic carbene (NHC) ligands. Subsequent abstraction of the chloride gave dicationic gold species that display Lewis acidic behavior. The synthesis of these complexes through OA provides direct experimental evidence for the involvement of such steps in dual gold/photoredox catalysis. The mild room temperature conditions and broad ligand scope also suggest that this method could be used to study the properties and reactivity of gold(III) species.
- Oxidative Addition to Gold(I) by Photoredox Catalysis: Straightforward Access to Diverse (C,N)-Cyclometalated Gold(III) Complexes,
Adrian Tlahuext-Aca, Matthew N. Hopkinson, Constantin G. Daniliuc, Frank Glorius,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201602649