New High-Energy-Density Materials
Trinitrotoluene or TNT has been considered as the standard measure for explosives for 100 years, although new high-energy-density materials or HEDMs outperform this substance in terms of explosion power, safety, but also environmental compatibility. In the journal Angewandte Chemie, Russian scientists present the synthesis of a highly interesting HEDM, which exhibits excellent energetic properties as well as a beautiful, butterfly-like structure.
Besides the pure performance aspect, research on new explosives has focused on finding substances that are less toxic to the environment, and less sensitive and thus safer, but still have high explosive power. Both the environmental aspect and explosiveness can be addressed by designing compounds with high nitrogen content but low carbon content, because nitrogen-rich substances decompose mainly into environmentally benign pure nitrogen, avoiding the formation of toxic nitrosamines. On the other hand, introducing a cyclic electron delocalization and alternating ionic charges in the molecule can add thermostability and high density.
High Nitrogen Content
A research team led by Aleksandr M. Churakov from the Russian Academy of Sciences, Moscow, has long studied the very promising compound tetrazino-tetrazine 1,3,6,8-tetraoxide (TTTO), which was theoretically calculated to combine very favorable parameters. They have now achieved its synthesis.
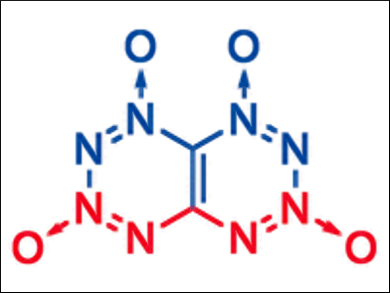
TTTO (pictured) is a completely symmetric compound composed of a bicyclic aromatic ring system. Eight of the ten atoms of the bicycle are nitrogen, so that the double ring system comprises two fused tetrazine rings in a butterfly-like arrangement. The oxidation of two of the four nitrogen atoms in each tetrazine ring with one oxygen atom, respectively, provides perfect oxygen balance and additional structural stabilization. Thus, this compound will decompose into pure nitrogen and carbon dioxide, and in addition, it is assumed to have excellent energetic characteristics.
Outperforming TNT
Of course, the synthesis of explosives needs great care. In the case of TTTO, the scientists developed a procedure involving ten steps. Having the new compound in hand, they start to characterize it to check if the theoretical properties are fulfilled. One of the critical practical requirements its sensitivity to thermal or mechanical treatment. “In the preliminary experiments, it did not detonate when rubbed in an agate mortar, but exploded in unglazed porcelain one,” the authors wrote on their initial studies.
Another requirement of new energetic materials is water resistance. In that respect, the authors admit that TTTO may be a bit weak. On the other hand, the chemical and energetic performances of new nitrogen-rich highly energetic compounds have to be carefully balanced. Therefore, TTTO serves as a particularly valid example of next-generation high-energy-density materials, which clearly outperform the traditional TNT standard.
- Synthesis of Tetrazino-tetrazine 1,3,6,8-Tetraoxide (TTTO),
Michael S. Klenov, Alexey A. Guskov, Oleg V. Anikin, Aleksandr M. Churakov, Yurii A. Strelenko, Ivan V. Fedyanin, Konstantin A. Lyssenko, Vladimir A. Tartakovsky,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201605611