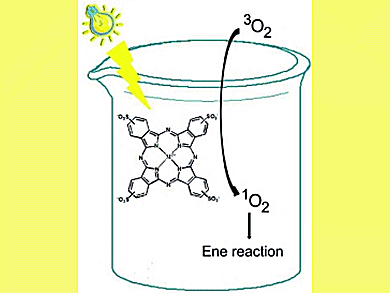
Scientists around Nicola d’Alessandro, University G. D’Annunzio of Chieti-Pescara, Pescara, Italy, and Salvatore Sortino, University of Catania, Catania, Italy, have investigated the capability of platinum, palladium and ruthenium sulfophthalocyanines (PtPcS, PdPcS and RuPcS) to act as singlet oxygen [1O2(1Δg)] photosensitizers in ene reactions in aqueous medium. For this they combined time-resolved and steady-state techniques.
Laser flash photolysis experiments with nanosecond time resolution revealed the population of the lowest excited triplet state in the case of PtPcS and PdPcS upon light excitation. In both cases, this transient is effectively quenched by molecular oxygen leading to the formation of 1O2(1Δg) with a quantum yield ΦΔ = 0.24, as unequivocally demonstrated by time-resolved near-infrared luminescence.
In contrast, RuPcS did not photosensitize 1O2(1Δg), in accordance with the lack of population of the precursor excited triplet state.
These metal–sulfophthalocyanines (MPcSs) were further tested in the ene reaction. In line with the photophysical results, PtPcS and PdPcS photosensitized the formation of hydroperoxide by 1O2(1Δg) addition to the target ,β-unsaturated carboxylic derivatives whereas RuPcS was totally inactive in this respect. Supporting the MPcSs on Amberlite® apparently made the ene reaction more rapid.
- Water-Soluble Transition-Metal-Phthalocyanines as Singlet Oxygen Photosensitizers in Ene Reactions,
Primiano D’Ambrosio, Lucia Tonucci, Nicola d’Alessandro, Antonino Morvillo, Salvatore Sortino and Mario Bressan,
Europ. J. Inorg. Chem. 2011.
DOI: 10.1002/ejic.201000784