Mechanically interlocked molecules, such as catenanes, rotaxanes, daisy chains, Borromean rings, and trefoil knots, rely on noncovalent bonding interactions to direct their assembly. How do these noncovalent bonding interactions affect the formation and stability of secondary structures in synthetic polymeric materials?
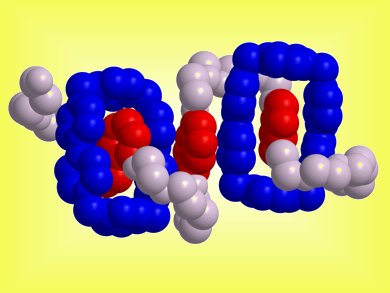
J. Fraser Stoddart and co-workers, Northwestern University, USA, have investigated a series of donor–acceptor oligorotaxanes. These were composed of tetraethylene glycol chains interspersed with three and five 1,5-dioxynaphthalene units and terminated by 2,6-diisopropylphenoxy stoppers as the dumbbells and cyclobis(paraquat-p-phenylene) molecules as the rings.
The secondary structures and conformations of these rotaxanes revealed a high degree of folding of the dumbbell thread. This folding arises from a combination of hydrogen bonds and π–π stacking between the π-electron-deficient bipyridinium units of the rings and the π-electron-rich 1,5-dioxynaphthalene units and polyether chains of the dumbbells.
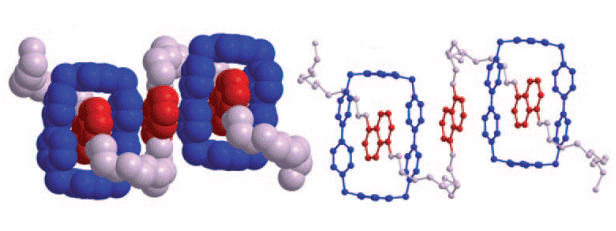
These results not only offer the possibility of creating new materials with elastic properties, but could also provide a better understanding of the nature of folding in biopolymers, such as proteins and nucleic acids.
Images: (c) Wiley-VCH
- Donor Acceptor Oligorotaxanes Made to Order
S. Basu, A. Coskun, D. C. Friedman, M. A. Olson, D. Benítez et al.,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201001822