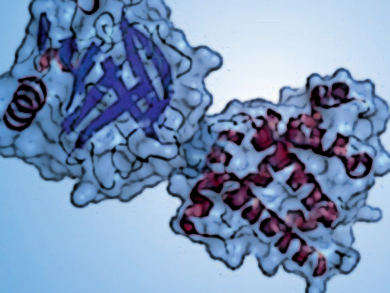
Gert von Helden, Fritz Haber Institute, Berlin, Germany, and colleagues have shown that the native structures of β-lactoglobulin and myoglobin, prototypical examples for β-sheet and helix-rich proteins, respectively, can be conserved in the absence of solvent. The target proteins were carefully transferred from buffered solution into the gas phase. There, mass and shape selection by ion-mobility-based mass spectrometry were performed, followed by spectroscopic investigation using the Fritz Haber Institute’s free-electron laser system.
The mass spectrometry (MS) results show that the ions are compact and have geometrical sizes that are similar to those of the native proteins in solution. the question whether their secondary structures also remained unaltered was answered using gas-phase IR spectroscopy. The results show a very good agreement with condensed-phase IR spectra, indicating that aspects of the proteins’ native secondary and tertiary structure can be conserved after the solvent is completely removed.
The study shows that sensitive mass spectrometry techniques, when properly applied, can be used to address problems relevant to structural biology.
- Retention of Native Protein Structures in the Absence of Solvent: A Coupled Ion Mobility and Spectroscopic Study,
Jongcheol Seo, Waldemar Hoffmann, Stephan Warnke, Michael T. Bowers, Kevin Pagel, Gert von Helden,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201606029