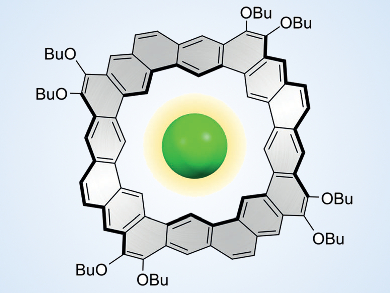
Several new molecular designs have recently been inspired by the infinite honeycomb pattern of graphene and its relevance to materials science. Nanometer-sized molecular fragments of this honeycomb, known as nanographenes, can be seen as “cut-outs” made from the graphene sheet. However, they are typically made by rational multistep synthesis. Their structures can be changed by doping with other elements, such as nitrogen, or by altering the hexagonal pattern of rings. Coronoids, crownlike fragments of graphene which contain large polygonal “holes” (example pictured), have attracted the attention of chemists, but are still difficult to prepare and to handle.
Dongho Kim, Yonsei University, Seoul, Republic of Korea, Marcin Stępień, University of Wrocław, Poland, and colleagues have synthesized octulene, the first coronoid hydrocarbon ring with a saddle-like distortion. The saddle shape of the molecule was established using the so-called “fold-in” method, which uses a coupling reaction to “stitch” the inner edge of the octulene molecule. The oxidation of the nickel(0) reagent in the fold-in process provides the energy for wringing the aromatic molecule out of planarity.
The distorted cavity of octulene showed an unexpected ability to bind chloride ions. This observation is remarkable because octulene bears no positive charge and is nonpolar. The interaction with the chloride ion takes place through extremely weak interactions with inner C–H bonds, which compete with the interaction between the chloride ions and the solvent benzene.
This discovery reveals the possibility of using preorganized hydrocarbon macrocycles as anion receptors, and offers a new insight into the thermodynamics of anion binding in nonpolar environments.
- Octulene: A Hyperbolic Molecular Belt that Binds Chloride Anions,
Marcin A. Majewski, Yongseok Hong, Tadeusz Lis, Janusz Gregoliński, Piotr J. Chmielewski, Joanna Cybińska, Dongho Kim, Marcin Stępień,
Angew. Chem. Int. Ed.2016.
DOI: 10.1002/anie.201608384