Widely used peptide bond forming processes that use traditional coupling reagents suffer from the formation of stoichiometric quantities of by-products that need to be separated from the product. This approach involves the generation of an electrophilic acyl unit, which undergoes nucleophilic attack by the amine. Importantly, sensitive amino acids are susceptible to racemization under these activation conditions.
André B. Charette and colleagues, Université de Montréal, Canada, have developed a chemical ligation process that uses dichlorosilanes to couple amines and acids. The team believes that both species react with the dichlorosilane reagent to generate a putative aminosilyl carboxylate moiety that undergoes spontaneous siloxane extrusion to produce the amide.
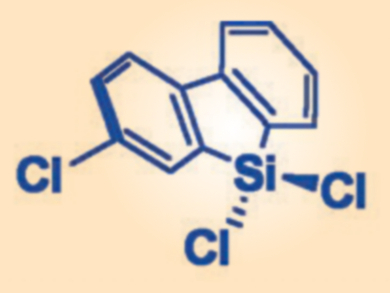
Optimization of the dichlorosilane reagent led to the identification of 9-silafluorenyl dichloride and its 3-chloro analogue (pictured) as optimal reagents for the amide-bond-forming process. Removal of the siloxane by-product is achieved by a simple filtration, leaving a solution of the pure peptide in the filtrate. The coupling of natural amino acids occurred in good yields and with no epimerization.
- 9-Silafluorenyl Dichlorides as Chemically Ligating Coupling Agents and Their Application in Peptide Synthesis,
Samuel J. Aspin, Sylvain Taillemaud, Patrick Cyr, André B. Charette,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201606120