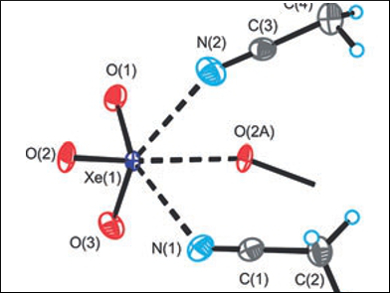
Xenon trioxide is an endothermic, shock-sensitive, and notoriously explosive compound which forms in the hydrolysis of XeF6 or XeF4. Despite the challenges associated with handling XeO3, Gary J. Schroblingen and colleagues, McMaster University, Hamilton, ON, Canada, have synthesized and isolated xenon trioxide adducts with one or two acetonitrile or propionitrile ligands (example pictured). The adducts were characterized by low-temperature single-crystal X-ray diffraction and Raman spectroscopy.
In the solid-state, Xe=O–Xe bridging interactions result in chain and network structures, in which the Lewis acidic xenon centers are consistently coordinated to three donor N or O atoms. The nature and strength of the Xe–O and Xe–N bonds of gas-phase models were extensively explored using a range of quantum-chemical methods, namely, natural bond orbital (NBO) analysis, quantum theory of atoms in molecules (QTAIM), molecular electrostatic potential surface (MEPS) analysis, and electron localization function (ELF) analysis. The Xe–N bonds were found to be primarily electrostatic, providing the first neutral xenon oxide adducts and a unique example of σ-hole bonding between a nitrogen base and a xenon oxide.
Unlike XeO3, the 1:2 adducts did not detonate when subjected to thermal or mechanical stresses, thus suggesting that the shock-sensitive behavior of XeO3 can be tamed when coordinated to appropriate ligands.
- Syntheses and Structures of Xenon Trioxide Alkylnitrile Adducts,
James T. Goettel, Kazuhiko Matsumoto, Hélène P. A. Mercier, Gary J. Schrobilgen,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201607583