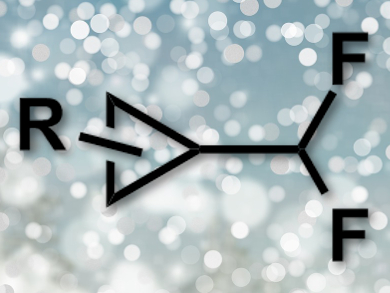
Difluoromethyl-substituted cyclopropanes can be useful functional groups, e.g., for biologically active molecules. However, there had only been elaborate multi-step syntheses for compounds with such groups so far, some using hazardous fluorinating reagents.
Rene M. Koenigs and colleagues, RWTH Aachen University, Germany, have developed the first catalytic one-step synthesis of difluoromethyl cyclopropanes from readily available reactants. The team first prepared difluoromethyl diazomethane in a continuous-flow process starting from difluoroethylamine, tBuONO, and acetic acid.
Then the researchers reacted this precursor with styrene derivatives in the presence of a rhodium(II) catalyst (Rh2esp2, esp = α,α,α’,α’-tetramethyl-1,3-benzenedipropionic acid) to give the desired product in one step. The reaction gives good isolated yields and tolerates substituted styrenes. Heterocyclic and non-aromatic substrates, in contrast, did not give the desired difluoromethyl cyclopropanes.
- Rhodium catalyzed synthesis of difluoromethyl cyclopropanes,
Katharina J. Hock, Lucas Mertens, Rene M. Koenigs,
Chem. Commun. 2016.
DOI: 10.1039/c6cc07745e