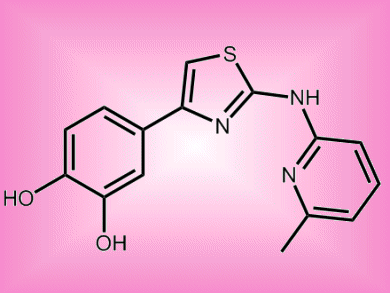
US researchers have identified 2-aminothiazoles as possible non-toxic lead compounds in the search for a pharmaceutical to treat prion diseases, such as Creutzfeldt-Jakob Disease (CJD). Prions are infectious agent composed of protein in a misfolded form. They duplicate themselves as proteins misfold in the brain leading to lethal deposits and holes in brain tissue.
Tests on the 2-aminothiazoles-based compounds in mice suggest that they can cross the blood-brain barrier and in laboratory tests show activity against abnormal protein accumulation. Protein misfolding is also present in Alzheimer’s disease, Huntington’s disease, Parkinson’s disease. Prion diseases are invariably fatal, and no viable treatments are currently available.
- 2-Aminothiazoles as Therapeutic Leads for Prion Diseases
A. Gallardo-Godoy, J. Gever, K. L. Fife, B. M. Silber, S. B. Prusiner, A. R. Renslo,
J. Med. Chem. 2011, 54(4), 1010–1021.
DOI: 10.1021/jm101250y