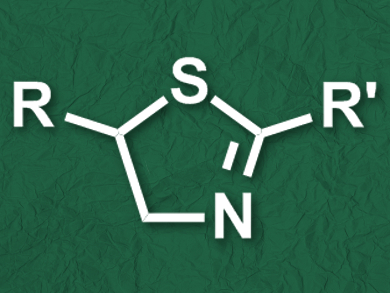
Thiazolines and their derivatives are common structures in natural products and bioactive compounds. They appear, for example, in the luminescent luciferin of fireflies or in antiretrovirals such as ritonavir. A commonly used method to synthesize thiazolines is the condensation of β-aminothiols with acids, esters, or nitriles. However, only a limited range of β-aminothiols are available, which limits the scope of the method.
Wei Li and colleagues, University of Toledo, OH, USA, have developed a one-pot strategy for the synthesis of thiazoline derivatives (pictured) starting from readily available alkenes and thioamides. The team first converted an alkene to the corresponding 1,2-dibromoalkane using LiBr and urea hydrogen peroxide (UHP). The nucleophilic addition of a thioamide to the dibromoalkane then led to the desired thiazoline derivative. Since both processes use the same solvent, namely acetonitrile (CH3CN), the synthesis can be performed as a one-pot procedure.
The reaction proceeds in good yields and tolerates a wide range of functional groups. The thiazoline products can be further transformed into thiazoles or β-aminothiols.
- One-Pot Strategy for Thiazoline Synthesis from Alkenes and Thioamides,
Nur-E Alom, Fan Wu, Wei Li,
Org. Lett. 2017.
DOI: 10.1021/acs.orglett.7b00079