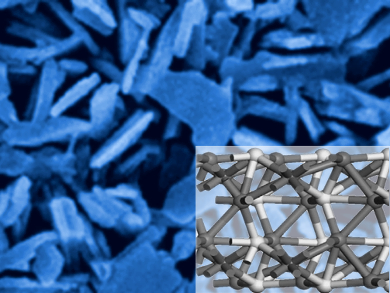
Splitting water into hydrogen and oxygen is key to a possible hydrogen economy that could replace fossil fuels. However, the high overpotential and sluggish kinetics of the oxygen evolution reaction (OER) make it difficult to realize this process in an efficient way. Catalysts that lower this overpotential are therefore needed.
Wen-Hua Zhang, An-Wu Xu, University of Science and Technology of China, Hefei, and colleagues have developed a one-step process to create iron–nickel sulfide nanosheets (pictured) on FeNi alloy foils (Fe–Ni3S2/FeNi) which show high catalytic activity for the OER. The team used a solvothermal process to directly grow Fe-doped Ni3S2 nanosheets on the FeNi alloy foils by reacting them with sulfide ions. The foil acts as support and current collector, and the nanosheets anchored on its surface have a high surface area and a large number of active sites for catalysis.
The resulting free-standing electrodes were tested in a water-splitting setup, and the researchers found that the material has a high catalytic activity and good stability for the OER in alkaline media. The overpotential is comparatively low at 282 mV at 10 mA cm−2. The team attributes these properties to the Fe-doping, which induces a disordered state in the nickel sulfide structure that changes its electronic properties and improves electron transport and catalytic activity.
- One-Step In Situ Growth of Iron-Nickel Sulfide Nanosheets on FeNi Alloy Foils: High-Performance and Self-Supported Electrodes for Water Oxidation,
Cheng-Zong Yuan, Zhong-Ti Sun, Yi-Fan Jiang, Zheng-Kun Yang, Nan Jiang, Zhi-Wei Zhao, Umair Yaqub Qazi, Wen-Hua Zhang, An-Wu Xu,
Small 2017.
DOI: 10.1002/smll.201604161