Hydrogen bonding is an important interaction, especially in biological systems such as DNA and proteins. Known strong hydrogen bond donors are OH groups, NH groups, and SH groups. C–H bonds usually cannot act as hydrogen bond donors due to a lack of polarity. However, the difluoromethyl group has been proposed to be a hydrogen bond donor.
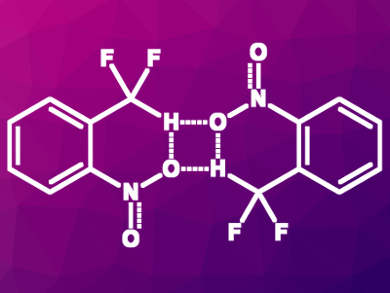
Stephen J. Lippard, Massachusetts Institute of Technology (MIT), Cambridge, USA, and colleagues have investigated hydrogen bonding interactions involving CF2H groups. The team studied the interactions in o-nitrophenol dimers and in o-nitro-α,α-difluorotoluene dimers (pictured) using NMR spectroscopy, infrared (IR) spectroscopy, X-ray crystallography, and quantum chemical calculations.
The team found evidence that supports the proposed hydrogen-bond donor character of CF2H groups. The CF2H group and the OH group, respectively, stabilize the o-nitrophenol and the o-nitro-α,α-difluorotoluene dimers and lead to very similar structures. The bonding energy of the OH-substituted dimer was calculated to be –3.5 kcal/mol and that of the CF2H-substituted variant is –3.1 kcal/mol. According to the researchers, CF2H groups could be used as an OH group surrogate in medicinal chemistry. CF2H could also be helpful for the design of molecules which disrupt other hydrogen bonds found in chemical biology.
- CF2H, a Hydrogen Bond Donor,
Chanan D. Sessler, Martin Rahm, Sabine Becker, Jacob M. Goldberg, Fang Wang, Stephen J. Lippard,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b04457