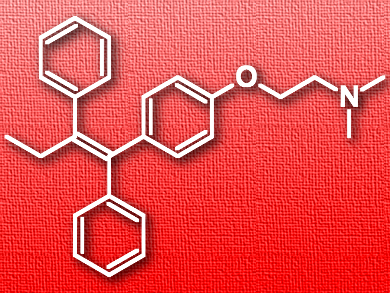
Tamoxifen (pictured) is an anticancer medication often used to treat breast cancer. Like many other drugs or drug candidates, it is poorly soluble in water, which hinders its absorption and limits its bioactivity. One possible approach to solve this problem is forming a complex between the pharmaceutically active molecule as the guest and a water-soluble macrocycle as the host. However, due to the selectivity of host–guest interactions, there is no macrocycle that is universally usable for different bioactive molecules.
Feihe Huang, Zhejiang University, Hangzhou, China, and colleagues have discovered a water-soluble pillar[6]arene which can increase the solubility of tamoxifen in water and improve its bioactivity. The pillar[6]arene is a macrocycle consisting of six arene groups, connected by CH2 units, with two carboxylate groups per arene. These charged groups make the macrocycle water-soluble, while its inner cavity is hydrophobic and can interact with tamoxifen.
The team characterized the resulting complex using 1H NMR, UV-Vis, and fluorescence spectroscopy and found clear evidence for host–guest interactions between tamoxifen and the pillar[6]arene. They also studied the water solubility of tamoxifen in the complex and found a remarkably good solubilizing effect.
The pillar[6]arene alone did not affect the viability of cancer cell colonies in vitro. The complex with tamoxifen, in contrast, showed cytotoxic effects after 12 hours of incubation. The complex was more effective than free tamoxifen, which the researchers attribute to the improved solubility caused by the macrocyclic host.
- Enhancing the solubility and bioactivity of anticancer drug tamoxifen by water-soluble pillar[6]arene-based host–guest complexation,
Liqing Shangguan, Qi Chen, Bingbing Shi, Feihe Huang,
Chem. Commun. 2017.
DOI: 10.1039/c7cc05305c