Michael P. Burke, Columbia University, New York, NY, USA, and Stephen J. Klippenstein, Argonne National Laboratory, Il, USA, have discovered a new class of chemical reactions: Three molecules each participate in the breaking and forming of chemical bonds.
Up to now, three classes of reactions have been considered: Unimolecular reactions (one reactant makes or breaks bonds); bimolecular reactions (two reactants collide and react); and termolecular association reactions (two reactants collide and combine, and then bump into a third molecule that takes some energy away to make it stable). A fourth class, chemically termolecular reactions, was first hypothesized by Cyril Hinshelwood and Nikolay Semenov in the 1920s and 30s (e.g., [1]) and was then considered unimportant.
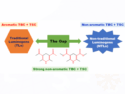
Using data from ab initio master equation and kinetic-transport simulations for the processes in the reaction of gases, for example, during combustion, the researchers showed that chemically termolecular reactions are major chemical pathways and that they impact flame propagation speeds, a measured rate of expansion of the flame front in a combustion reaction. The reaction is enabled by an “ephemeral collision complex”, formed from the collision of two molecules (e.g., H + O2). It lives long enough to collide with a third, reactive molecule (e.g. H radical), yielding in a “chemically termolecular reaction” product.
This reaction class probably plays an important role in our daily lives. According to the researchers, it is found, among others, in combustion engines, in rocket propellants, or in lightning in the earth’s atmosphere. In the engines and rocket engines, this three-partner reaction could influence the spread of flames and heat.
- Ephemeral collision complexes mediate chemically termolecular transformations that affect system chemistry,
Michael P. Burke, Stephen J. Klippenstein,
Nature Chem. 2017.
DOI: 10.1038/nchem.2842
[1] Cyril Norman Hinshelwood, Thomas Edward Green, J. Chem. Soc. 1926, 129, 730–739. https://doi.org/10.1039/JR9262900730