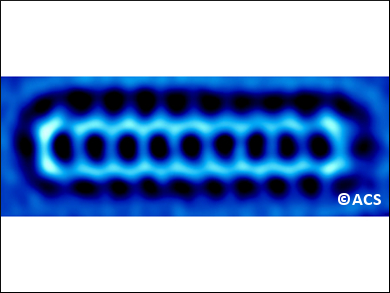
Acenes are the narrowest graphene nanoribbons—or polycyclic aromatic hydrocarbons that consist of linearly fused benzene rings. Their electromagnetic properties make them ideal for applications in electronic devices, such as transistors and LEDs, as well as in spintronics and plasmonics.
Szymon Godlewski, Jagiellonian University, Krakow, Poland, Antonio M. Echavarren, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and colleagues have synthesized nonacene, the second longest acene ever prepared. They managed to study the electronic properties of this highly reactive acene for the first time and obtained several high-resolution microscopy images of the molecule (example pictured). The researchers deposited a partially saturated precursor on a gold surface. Then, using the tip of a microscope that combines scanning tunneling and atomic force techniques, they triggered the dehydrogenation to fully aromatic nonacene.
The electronic properties of nonacene were analyzed using scanning tunneling spectroscopy. The researchers also were able to obtain electron density maps of a range of nonacene’s molecular orbitals, which agree well with theoretical calculations. The measurements verify the expected decrease in the HOMO-LUMO gap in the acene series as the number of fused rings increases. This could make nonacene a very good low-bandgap semiconductor.
This on-surface method for the synthesis of acenes opens up new possibilities for the preparation of longer graphene nanoribbons and for the functionalization of existing ones towards more advanced functional materials.
- Nonacene Generated by On-Surface Dehydrogenation,
Rafal Zuzak, Ruth Dorel, Mariusz Krawiec, Bartosz Such, Marek Kolmer, Marek Szymonski, Antonio M. Echavarren, Szymon Godlewski,
ACS Nano 2017.
DOI: 10.1021/acsnano.7b04728