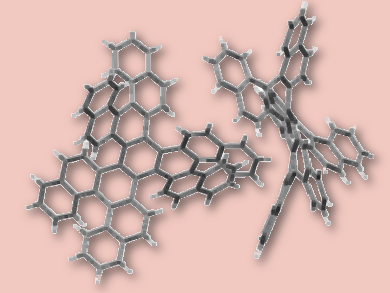
A challenge in current research is to use chirality to fine-tune and promote properties of organic materials, such as their physical, supramolecular, photophysical, conductive, chiroptical, molecular recognition and switching properties. Yoann Coquerel, Marc Gingras, and colleagues, Aix Marseille University, CNRS, Marseille, France, have synthesized a chiral propeller-shaped D3-symmetric nanographene. The polyaromatic hydrocarbon molecule embeds six [5]helicene units. it is synthesized in one step starting from 7,8-dibromo[5]helicene by Yamamoto nickel(0) couplings.
Single-crystal X-ray diffraction analysis shows that the triphenylene core is extremely distorted. The three internal [5]helicenes show a strong helical stretch. This leads to abnormally large interplanar angles between their two terminal rings, resulting in an accumulation of dense helical strain. This makes the molecule configurationally stable. The team produced it in enantiopure forms by chiral HPLC techniques.
According to the researchers, this short, effective synthesis to chiral, configurationally stable, propeller-shaped nanographenes opens up new avenues toward future applications in materials science, chiroscience, and nanoscience.
- A Chiral Nanographene Propeller Embedding Six Enantiomerically Stable [5]Helicene Units,
Veronika Berezhnaia, Myriam Roy, Nicolas Vanthuyne, Marco Villa, Jean-Valère Naubron, Jean Rodriguez, Yoann Coquerel, Marc Gingras,
J. Am. Chem. Soc. 2017.
https://doi.org/10.1021/jacs.7b07622


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)