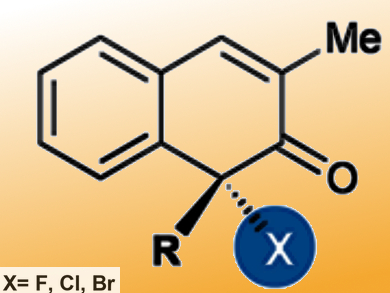
Axially chiral compounds are widely used in asymmetric synthesis, either as chiral catalysts or as chiral ligands. However, using these compounds in other transformations, such as chirality conversion reactions, has been less developed.
Dongxu Yang and Rui Wang, Lanzhou University and Lanzhou Institute of Chemical Physics, China, and colleagues have reported a simple axial-to-central chirality conversion reaction. The axially chiral naphthols, derived from BINOLs (1,1’-2-naphthols), react with commercially available halogenation reagents to give chiral α-halogenated ketones (pictured) in good yields and generally excellent enantioselectivities.
The fluorination reaction uses NFSI (N-fluorobenzenesulfonimide) as a reagent and CuBr as a catalyst and proceeds in tBuOMe at 0 °C in the presence of tBuOLi. The chlorination reaction takes place in THF at room temperature or 40 °C using AlCl3 as a catalyst. The asymmetric bromination occurs in CH2Cl2 at room temperature without a catalyst, but only moderate ee is obtained. The halogenated products can be further transformed to other useful compounds, such as a chiral azide or a chiral epoxide. The researchers have provided an enantioselective and mild method to transfer axial chirality to central chirality.
- Asymmetric Dearomative Halogenation of β-Naphthols: The Axial Chirality Transfer Reaction,
Pengxin Wang, Jie Wang, Linqing Wang, Dan Li, Kezhou Wang, Yuyang Liu, Haiyong Zhu, Xihong Liu, Dongxu Yang, Rui Wang,
Adv. Synth. Catal. 2017.
https://doi.org/10.1002/adsc.201700745