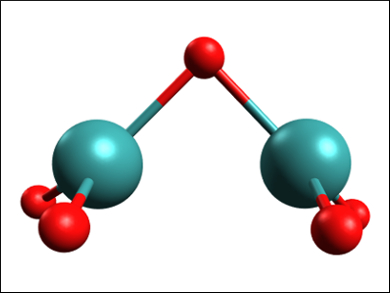
Technetium is the lightest element without any stable isotopes. It is produced by nuclear fission. Immobilizing and cleaning up technetium from nuclear fuel and decommissioned nuclear complexes usually involves mixing it into borosilicate glass. However, due to the high temperatures during this process, a large part of the radioactive Tc can be transformed into volatile species. One of these species, a volatile technetium oxide called “tech red”, has remained structurally uncharacterized for over 50 years.
Keith V. Lawler, Paul M. Forster, University of Nevada, Las Vegas, USA, and colleagues have used density functional theory (DFT) calculations to find the most likely structure of “tech red”. The team calculated the structures and relative energies of different isomers of Tc2O4, Tc2O5, and Tc2O6. They also simulated UV-Vis spectra for these compounds which could be compared with that of “tech red”, which has a characteristic absorption maximum at 505 nm.
The spectral data narrowed down the possible structures to three candidates, of which a Tc2O5 molecule with a bridging oxygen (pictured) is the most stable compound. The average oxidation state of Tc in this molecule is in agreement with redox titrations, and the molecule can dimerize and polymerize, which has also been postulated for “tech red”. According to the researchers, this close match with experimental results makes the oxygen-bridged Tc2O5 the most likely compound to be involved in forming “tech red”.
- Unraveling the Mystery of “Tech Red” – a Volatile Technetium Oxide,
Keith Vanoy Lawler, Bradley Childs, Kenneth R. Czerwinski, Alfred P. Sattelberger, Frederic Poineau, Paul M. Forster,
Chem. Commun. 2018.
https://doi.org/10.1039/c7cc09191e