Chiral amines are very important building blocks in organic synthesis. The transition-metal-catalyzed asymmetric reductive amination of ketones is the most attractive and straightforward approach to synthesize chiral amines. However, the method is only effective for certain ketones or for synthesizing secondary amines. This is due to the incompatibility of transition-metal hydrides with ketones and the coordination of the amines to the metal catalyst.
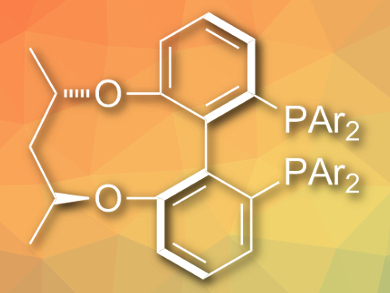
Qin Yin, Xumu Zhang, and colleagues, Southern University of Science and Technology, Shenzhen, China, have developed an efficient method to directly synthesize chiral primary amines from simple ketones using Ru(OAc)2 with a C3-TunePhos-type ligand (pictured) as a catalyst [1]. NH4OAc is used as an amine source and H2 (55 bar) as the reducing reagent. The reactions are carried out in trifluoroethanol at 80 °C to give the products in excellent yields and enantioselectivities.
Recently, Thomas Schaub, Catalysis Research Laboratory, Heidelberg, and BASF SE, Ludwigshafen, Germany, and colleagues had achieved the same goal using NH3 (6 bar) as an amine source, [Ru(Cl)H(CO)(PPh3)3] as a catalyst, and binaphane as a ligand under H2 (40 bar) in the presence of NaPF6 and NH4I in toluene at 120 °C [2].
Yin, Zhang, and colleagues demonstrated the practicability of their method by the scalable synthesis of key intermediates of three drug molecules. Schaub and colleagues investigated the reaction mechanism with the help of density functional theory (DFT) calculations and proposed a possible pathway. Unfortunately, both methods gave poor results for dialkylketones.
- [1] Asymmetric Synthesis of Chiral Primary Amines by Ruthenium-Catalyzed Direct Reductive Amination of Alkyl Aryl Ketones with Ammonium Salts and Molecular H2,
Xuefeng Tan, Shuang Gao, Weijun Zeng, Shan Xin, Qin Yin, Xumu Zhang,
J. Am. Chem. Soc.2018, 140, 2024–2027.
https://doi.org/10.1021/jacs.7b12898 - [2] Direct Asymmetric Ruthenium-Catalyzed Reductive Amination of Alkyl–Aryl Ketones with Ammonia and Hydrogen,
Joan Gallardo-Donaire, Marko Hermsen, Jedrzej Wysocki, Martin Ernst, Frank Rominger, Oliver Trapp, A. Stephen K. Hashmi, Ansgar Schäfer, Peter Comba, Thomas Schaub,
J. Am. Chem. Soc. 2017, 140, 355–361.
https://doi.org/10.1021/jacs.7b10496