Ketones are important synthetic intermediates in organic chemistry. One approach for their synthesis is the oxidative coupling of alkenes with organic radicals. However, the range of radicals which can be used for this reaction is limited. For example, aliphatic heterocycles are difficult to introduce using this method. This is due to the fact that they have weak C–H bonds which can be degraded by commonly used radical initiators.
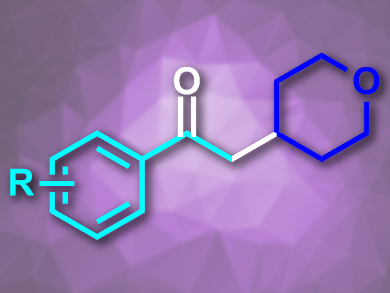
Frank Glorius and colleagues, University of Münster, Germany, have developed a strategy for the oxidative coupling of light-generated alkyl radicals with styrenes that has a high functional group tolerance. The team used a photoinduced reduction of N-(acyloxy)phthalimides under visible light, catalyzed by fac-Ir(ppy)3 (ppy = 2-phenylpyridine), to generate alkyl radicals. These were coupled with a range of styrenes using dimethyl sulfoxide (DMSO) as an oxidant to give the desired α-alkyl-acetophenones (example pictured).
The reaction proceeds in good yields at room temperature and needs only low photocatalyst loadings. The use of N-(acyloxy)phthalimides as sources of organic radicals avoids the degradation of weak C–H bonds in the alkyl substrates and, thus, improves the reaction’s functional group tolerance.
- Visible-Light-Mediated Synthesis of Ketones by the Oxidative Alkylation of Styrenes,
Adrian Tlahuext-Aca, R. Aleyda Garza-Sanchez, Michael Schäfer, Frank Glorius,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b00272