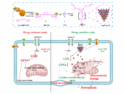
Photoelectrochemical water splitting using solar energy is a useful strategy to produce hydrogen for use as a fuel. The process requires a suitable semiconductor, most often inorganic materials. However, synthetic organic/polymer semiconductors are promising due to their abundance, tunable band structures, low production cost, and environmental sustainability.
Graphitic carbon nitride (g-C3N4) and conjugated carbon-rich polymers have been successfully used for direct solar water reduction, suggesting that carbon-rich materials can be suitable for this reaction. In particular, sp-hybridized (acetylenic) carbon-rich materials have potential as photocatalysts.
Xinliang Feng, Dresden University of Technology, Germany, and colleagues have developed a simple way of preparing acetylenic carbon-rich nanofibers. The team used a metallic copper wafer as a catalyst to induce a Glaser polycondensation of 1,3,5-triethynylbenzene in solution. The resulting poly(1,3,5-triethynylbenzene) (PTEB) nanofibers can be transferred to a variety of substrates, e.g., graphite foils, silicon dioxide, glass, or titanium plates.
The PTEB nanofibers on conductive substrates can be used as high-performance metal-free photocathodes for photoelectrochemical (PEC) devices that generate hydrogen from water. The diversity of terminal alkynes allows the rational design of a broad set of acetylenic carbon-rich materials with controlled optical and electronic properties. The researchers demonstrated this possibility by the introduction of thieno[3,2-b]thiophene segments into the PTEB nanofibers, which resulted in a 100 nm red-shift in light absorption and two-fold enhancement in photocurrent. The resulting performance is comparable with state-of-the-art metal-free photocathodes.
- Copper-surface-mediated synthesis of acetylenic carbon-rich nanofibers for active metal-free photocathodes,
Tao Zhang, Yang Hou, Volodymyr Dzhagan, Zhongquan Liao, Guoliang Chai, Markus Löffler, Davide Olianas, Alberto Milani, Shunqi Xu, Matteo Tommasini, Dietrich R. T. Zahn, Zhikun Zheng, Ehrenfried Zschech, Rainer Jordan, Xinliang Feng,
Nat. Commun. 2018.
https://doi.org/10.1038/s41467-018-03444-0