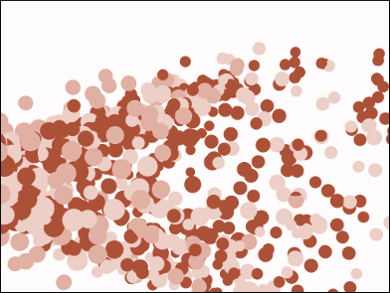
Metal nanoparticles (NPs) have useful properties for applications in, e.g, catalysis, optics, or medicine. These properties strongly depend on the particles’ size and shape. Thus, finding improved ways to prepare monodisperse NPs (i.e., NPs with a narrow size distribution) is important. Existing approaches for the synthesis of monodisperse copper NPs, for example, use harmful reducing agents in dilute solutions.
Takanari Togashi, Masato Kurihara, and colleagues, Yamagata University, Japan, have developed a method to synthesize monodisperse Cu NPs based on the thermal decomposition of an oleylamine-coordinated Cu oxalate complex in oleylamine in air without a reducing agent. The team prepared the complex by combining copper oxalate, oleylamine, and methanol in solution and removing the product by centrifugation. The complex was then dried, mixed with oleylamine, and heated to prepare the Cu NPs. During this process, the oxalate was converted to CO2 gas.
The size distribution of the resulting NPs depends strongly on the decomposition temperature and becomes narrower with increasing temperature. NPs synthesized at 260 °C have an average diameter of 13.8 nm. The NPs were obtained in high yields of about 90 %. According to the researchers, the developed thermal decomposition of alkylamine-oxalate complexes is a useful approach for the synthesis of monodisperse metal NPs.
- Solvent-free synthesis of monodisperse Cu nanoparticles by thermal decomposition of an oleylamine-coordinated Cu oxalate complex,
Takanari Togashi, Masato Nakayama, Atsuki Hashimoto, Manabu Ishizaki, Katsuhiko Kanaizuka, Masato Kurihara,
Dalton Trans. 2018.
https://doi.org/10.1039/c8dt00345a