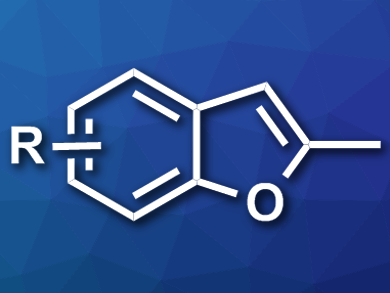
Benzofuran structures occur in many pharmaceutically active compounds. Their synthesis can require catalysts based on expensive metals or substrates that are difficult to obtain.
Rugang Fu and Zheng Li, Northwest Normal University, Lanzhou, China, have developed a synthesis of methyl-substituted benzofurans (example pictured) that uses inexpensive and readily available reagents. The team combined salicylaldehyde p-tosylhydrazones with calcium carbide as an acetylene source, CuCl as a catalyst, and KOtBu as a base in dimethylformamide (DMF) at 90 °C.
The reaction gives the desired benzofurans in good yields and tolerates a variety of substituents at the aromatic ring. In addition, 2,3-dimethylbenzofuranes could be synthesized under similar conditions starting from 2-hydroxylacetophenone p-tosylhydrazones. The reaction is cost-effective and highlights the potential of calcium carbide as an acetylene replacement in organic synthesis.
- Direct Synthesis of 2-Methylbenzofurans from Calcium Carbide and Salicylaldehyde p-Tosylhydrazones,
Rugang Fu, Zheng Li,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b00676