Sodium-ion batteries (NIBs) are a promising alternative to lithium-ion batteries for advanced electrical storage, which is important due to the limited natural abundance of lithium. However, the application of NIBs is still hindered by two drawbacks: Firstly, the bigger size of the Na+-ions, which lead to a complex multi-phasic behavior at the cathode detrimental to long-term durability. Secondly, the voltage of the sodium couples is approximately 0.5 V less than That of lithium couples, which leads to a lower energy density.
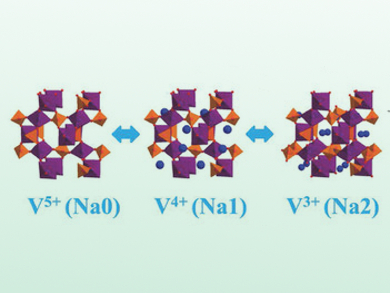
M. Stanley Whittingham, Binghamton University, NY, USA, and colleagues have used KVOPO4 as cathode material for NIBs. Vanadium has multiple redox states (+III to +V) that can be fully activated by the sodium ions in a polyhedral vanadyl phosphate phase. The structural framework allows the intercalation of 1.66 Na+ per formula unit. By this double activation of vanadium, the reduced voltage of the sodium couples is sufficiently compensated.
Structural characterization by X-ray absorption spectroscopy shows that there are only minor volume changes upon Na+-ion intercalation. With KVOPO4 as a cathode material, the team achieved a high energy density of 600 Wh kg–1 that exceeds the capacity of current cathodes.
- KVOPO4: A New High Capacity Multielectron Na-Ion Battery Cathode,
Jia Ding, Yuh-Chieh Lin, Jue Liu, Jatinkumar Rana, Hanlei Zhang, Hui Zhou, Iek-Heng Chu, Kamila M. Wiaderek, Fredrick Omenya, Natasha A. Chernova, Karena W. Chapman, Louis F. J. Piper, Shyue Ping Ong, M. Stanley Whittingham,
Adv. Energy Mater. 2018.
https://doi.org/10.1002/aenm.201800221