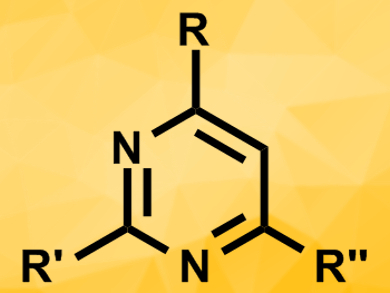
Pyrimidines are heteroaromatic compounds and commonly found as a substructure in bioactive molecules. They are usually synthesized from amidines and 1,3-dicarbonyl compounds. Finding synthesis methods that use cheaper and more readily available reactants could be very useful for the economical production of pyrimidines.
Jianyu Dong, Yongbo Zhou, and colleagues, Hunan University, Changsha, China, have developed a cost-effective synthesis of functionalized pyrimidines (pictured) that starts from inexpensive and structurally diverse reagents, i.e., ketones and nitriles, and tolerates a wide range of functional groups. The team used nitriles as electrophiles and combined them with ketones in the presence of NaOH as a base and CuCl2 as a catalyst at 120 °C.
The desired pyrimidines are obtained in good to excellent yields and the reaction tolerates, e.g., ethers, thioethers, amines, and alcohols. The proposed reaction mechanism involves an enolic intermediate, which attacks the nitrile to give an imine. The imine is isomerized and dehydrated to give an enaminone ion, which attacks a second nitrile molecule. A cyclocondensation then gives the final product. According to the researchers, the method could be useful in both chemical research and industry.
- Cyclization of Ketones with Nitriles under Base: A General and Economical Synthesis of Pyrimidines,
Lebin Su, Kang Sun, Neng Pan, Long Liu, Mengli Sun, Jianyu Dong, Yongbo Zhou, Shuang-Feng Yin,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b01324