In nature, closed environments such as cell organelles allow chemical reactions to take place under ambient conditions and in a controlled manner. This has inspired chemists to perform reactions in environments such as micelles, mesoporous materials, microemulsions, or gels.
David Díaz Díaz, University of Regensburg, Germany, and Institute of Advanced Chemistry of Catalonia (IQAC−CSIC), Barcelona, Spain, and colleagues have used a gel network to enable air-sensitive photoredox catalysis reactions under ambient conditions. In this approach, the gel replaces the conditions of an inert atmosphere. It hampers the diffusion of oxygen and prevents it from interfering with the reaction.
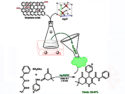
The team studied, e.g., the effect of a gel environment on C–C bond forming reactions between aryl halides and trapping agents (for example, N-methylpyrrole) with rhodamine-6G as a photocatalyst. They added N,N’-bis(octadecyl)-L-Boc-glutamic diamide to the reaction mixture as a gelator and compared the results with the same reaction in an aerated solution. In a “normal” solution in air, the reaction is ineffective (yield below 5 %). In the gel, the yield was improved to 73 %.
According to the researchers, the yields in a gel environment are comparable to those in solution under inert conditions. The gel is easy to remove after the reaction.
- Air-Sensitive Photoredox Catalysis Performed under Aerobic Conditions in Gel Networks,
Marleen Häring, Alex Abramov, Keisuke Okumura, Indrajit Ghosh, Burkhard König, Nobuhiro Yanai, Nobuo Kimizuka, David Díaz Díaz,
J. Org. Chem. 2018.
https://doi.org/10.1021/acs.joc.8b00797