Coordination polymers (CPs) are composed of metal cations linked by organic ligands and can form one-, two- or three-dimensional structures. Rare-earth-based CPs have been used for a wide range of applications, such as gas storage and separation, sensing, and catalysis. Lina M. Aguirre-Díaz, M. Ángeles Monge, Consejo Superior de Investigaciones Científicas, Madrid, Spain, and colleagues have found that this type of CP can be used as a green photocatalyst for the synthesis of imines at room temperature.
The team has designed five rare-earth CPs with ligands as light-harvesting antennas and catalytically active metal centers. They combined either La, Nd, Sm, Ho, or Er with the ligand 9,9′-spirobi[9H-fluorene]-2,2′-dicarboxylic acid (H2sfdc) in water or a water/ethanol mixture to form the CPs. The CPs based on La, Nd, Sm, and Ho form one-dimensional chains, while the CP based on Er forms a three-dimensional network.
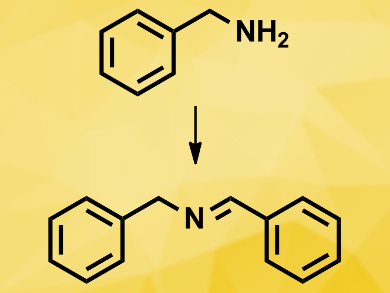
The catalysts performed well in the photocatalytic oxidation of amines to imines (example pictured), with conversion rates of up to 76 % and selectivities of up to than 99 %. They could be recovered and used without any material loss and any loss in the selectivity towards the desired imine product.
- Efficient Rare-Earth-Based Coordination Polymers as Green Photocatalysts for the Synthesis of Imines at Room Temperature,
Lina M. Aguirre-Díaz, Natalia Snejko, Marta Iglesias, Félix Sánchez, Enrique Gutiérrez-Puebla, M. Ángeles Monge,
Inorg. Chem. 2018.
https://doi.org/10.1021/acs.inorgchem.8b00465