Cubane is a cube-shaped hydrocarbon. As with many other organic compounds, making cubane analogues with heavier elements is difficult.
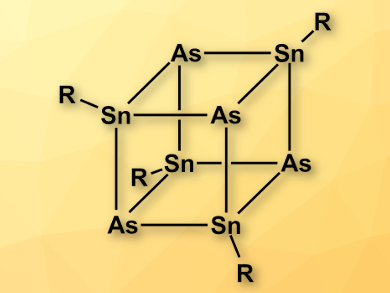
Alexander Hinz and Jose M. Goicoechea, University of Oxford, UK, have synthesized a heterocubane of the type [RSnAs]4 (pictured, R = 2,6-dimesitylphenyl). The team combined a 2,6-dimesitylphenyl-substituted chlorostannylene and salts of the heavy cyanate analogue AsCO– at room temperature in toluene to give the heterocubane. The compound is the first cubane containing SnIV in which the organic substituents are located on the tin atoms. The team characterized [RSnAs]4 using 119Sn NMR spectroscopy and X-ray crystallography.
Attempts to synthesize the corresponding phosphorus-containing cubane [RSnP]4 in an analogous manner were not successful. A stannylenyl-phosphaketene (RSnPCO) was formed initially, but decomposed spontaneously and gave a mixture of products instead of the heterocubane.
- The heterocubane [TerSnAs]4,
Alexander Hinz, Jose M. Goicoechea,
Dalton Trans. 2018.
https://doi.org/10.1039/c8dt01993b