Compounds of the type PXnH3–n (X = halogen, n = 1,2) are often too unstable to isolate, with the exception of PF2H, which is stable as a liquid. PFH2 and PClH2, for example, are short-lived and could only be detected spectroscopically in the gas phase. Monoiodophosphine PIH2 and diiodophosphine PI2H were detected in reaction mixtures, but have not been isolated so far. N-heterocyclic carbenes (NHCs) have previously been used to stabilize otherwise elusive inorganic species.
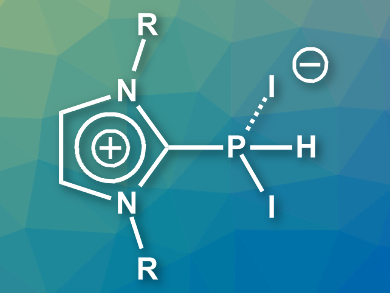
Dietrich Gudat, University of Stuttgart, Germany, and colleagues have synthesized NHC-adducts of PI2H which can be isolated. The team reacted PH-substituted imidazolylidenes (NHC=PH) with molecular iodine at –78 °C to prepare the desired adducts (example pictured). The adducts were obtained in yields of 74–94 % and are stable in a dry atmosphere.
The compounds were characterized using X-ray crystallography and 31P NMR spectroscopy, among other methods. The adducts have two P–I bonds of different lengths. This points to a description as charge-transfer complexes between cationic phosphines, [NHC-PHI]+, and iodide. This interpretation was supported by the results of density functional theory (DFT) calculations.
- Isolable N-heterocyclic carbene adducts of the elusive diiodophosphine,
Mario Cicac-Hudi, Simon Schlindwein, Christoph M Feil, Martin Nieger, Dietrich Gudat,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc03972k