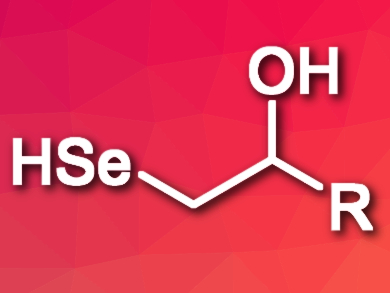
Alkylselenols are difficult to prepare because they have a tendency to decompose quickly and, e.g., form diselenides. This is due to the fact that the SeH group is very easy to oxidize.
Damiano Tanini, Antonella Capperucci, and colleagues, University of Florence, Italy, have developed a straightforward synthesis of β-hydroxy-functionalized alkylselenols (pictured), which were found to be unexpectedly stable. Epoxides were treated with the bis(trimethylsilyl)-selenide (Me3Si)2Se under controlled conditions, which allowed the team to avoid the formation of diselenides. Tetra-n-butylammonium fluoride (TBAF) was used as a catalyst and the reaction was performed at –15 °C. To prevent side reactions, the reaction was stopped after a few minutes by adding citric acid.
The desired β-hydroxy selenols were obtained in high yields and characterized using 1H and 77Se NMR spectroscopy. The researchers attribute the compounds’ relative stability to hydrogen-bond interactions between the SeH and OH groups. This hypothesis was supported by density functional theory (DFT) calculations.
- A Straightforward Access to Stable β-Functionalized Alkyl Selenols,
Damiano Tanini, Caterina Tiberi, Cristina Gellini, Pier Remigio Salvi, Antonella Capperucci,
Adv. Synth. Catal. 2018.
https://doi.org/10.1002/adsc.201800602