Amide bonds are important, e.g., in biological systems, drugs, agrochemicals, and polymers. Usually, amides are synthesized by coupling amines with activated carboxylic acid derivatives. However, the reactants used to activate carboxylic acids are often toxic and can usually not be recovered and reused.
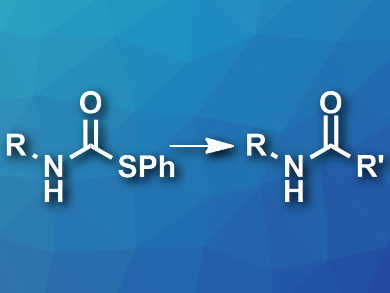
Bert U. W. Maes, University of Antwerp, Belgium, and colleagues have developed a greener approach to the synthesis of amides. The team used readily accessible S-phenyl thiocarbamate derivatives (pictured left) as starting materials and coupled them with Grignard reagents in 2-methyl-tetrahydrofuran at room temperature. An oxidative workup using an aqueous ammonium hydroxide solution under an oxygen atmosphere then gave the desired secondary amides (pictured right) and diphenyl disulfide (PhSSPh).
The diphenyl disulfide product can be oxidized to S-phenyl benzenethiosulfonate, which can then be reused to synthesize thiocarbamates as the starting materials for further amide syntheses. According to the researchers, the method can be used for the preparation of amides which are not accessible via other approaches and the recovery of the thiophenolate leaving group as diphenyl disulfide lowers waste production.
- Synthesis of Secondary Amides from Thiocarbamates,
Pieter Mampuys, Eelco Ruijter, Romano V. A. Orru, Bert U. W. Maes,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b01654