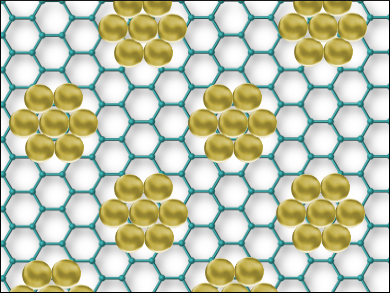
Hexagonal boron nitride (h-BN) is a 2D material best known for its interesting electronic properties. However, it can be also used as a template to build 2D superlattices. A superlattice is a periodic structure of layers of two or more materials (example pictured).
Moritz Will, University of Cologne, Germany, Nicolae Atodiresei, Forschungszentrum Jülich, Germany, and colleagues have found that h-BN on an Ir(111) surface can be used as a template for Ir, C, and Au cluster superlattices. The researchers carried out experiments under an ultrahigh vacuum and used sputtering methods to create h-BN islands from borazine on top of Ir(111) surfaces. To form the clusters, Ir or C atoms were then deposited onto the h-BN substrates by sublimation, whereas Au was evaporated from a so-called Knudsen cell and deposited on the h-BN islands.
The team used scanning tunneling microscopy (STM) to study the resulting superlattices. Depending on the experimental conditions, the experiments gave superlattices with an average cluster size of between 6 and 175 atoms. It was found that the superlattices are stable up to 1350 K. This is attributed to the clusters binding to the h-BN with strong cluster atom–boron bonds. This, in turn, reinforces the bonds between the h-BN nitrogen atoms and the Ir substrate, leading to a highly stable superlattice. These nanocluster superlattices could be used for nanocatalysis and nanomagnetism applications.
- A Monolayer of Hexagonal Boron Nitride on Ir(111) as a Template for Cluster Superlattices,
Moritz Will, Nicolae Atodiresei, Vasile Caciuc, Philipp Valerius, Charlotte Herbig, Thomas Michely,
ACS Nano 2018.
https://doi.org/10.1021/acsnano.8b02127