Ionic liquids often have a high electrochemical and thermal stability. This allows them to be used as solvents in deposition processes. In such processes, thermally sensitive precursor compounds decompose under high temperatures and form the desired materials. Incorporating the precursors directly in an ionic liquid would allow solvent-free deposition reactions. Such solvent-free methods eliminate the risk for contaminating the products with the solvent, which is especially important for metal sulfides, such as cadmium sulfide thin films, used in sensitive electronic devices (e.g., solar cells).
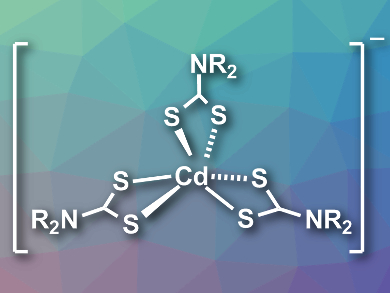
Lauren K. Macreadie and Anthony S. R. Chesman, CSIRO Manufacturing, Clayton, Australia, Craig M. Forsyth and David R. Turner, Monash University, Clayton, Australia, have synthesized the first cadmium-based ionic liquids to be used as cadmium sulfide precursors. The team combined bulky cations, such as tetramethylammonium, tetrapropylammonium, or 1-butyl-methylpyrrolidinium, with anionic cadmium dithiocarbamate complexes (pictured, R = iBu, nPr, Me, or Bu). Single-crystal X-ray diffraction showed that the anions in the resulting products are cadmium complexes with a distorted octahedral geometry.
The team studied the thermal decomposition of the thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The salts with 1-butyl-1-methylpyrrolidinium cations have melting points (ca. 64–67 °C) below the temperature needed to induce thermolysis and form CdS (ca. 150–167 °C). This allows these ionic liquids to be used as liquid CdS precursors in solvent-free depositions.
- Cadmium tris(dithiocarbamate) ionic liquids as single source, solvent-free cadmium sulfide precursors,
Lauren K. Macreadie, Craig M. Forsyth, David R. Turner, Anthony S. R. Chesman,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc03737j