Fossil resources are used as a feedstock for many important processes in the chemical industry—however, they are limited. CO2, in contrast, could be used as a renewable feedstock for the production of chemicals. CO2 hydrogenation could provide fuels as well as chemicals such as methanol, olefins, or aromatics. C1 compounds such a methane or methanol can already be selectively synthesized from CO2 and H2. A selective hydrogenation of CO2 to give aromatics, in contrast, had not been achieved so far.
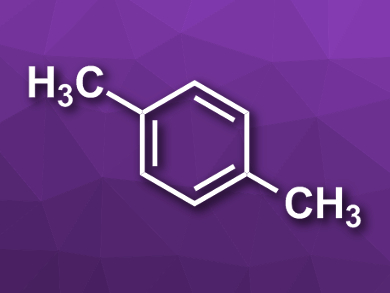
Wenliang Zhu, Zhongmin Liu, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and colleagues have developed a composite catalyst made from the oxide ZnAlOx and the zeolite H-ZSM-5 for the selective synthesis of aromatics (example pictured) from CO2 and H2. The team synthesized the ZnAlOx using a co-precipitation method. The zeolite was prepared from commercially available Na-ZSM-5 by ion exchange. The composite catalyst was then prepared by physical mixing of the two components in a 1:1 ratio.
Hydrogenation reactions were then performed over the composite catalyst using a gas mixture of CO2, H2, and argon (H2/CO2/Ar = 3/1/0.2) at a combined pressure of 3 MPa and at a temperature of 593 K. The team achieved up to 9 % CO2 conversion with a selectivity of aromatics among the carbon products (excluding CO) up to 74 %. The CH4 selectivity was only 0.4 %. According to the proposed mechanism, intermediates such as methanol and dimethyl ether are first formed on the ZnAlOx surface and then transmitted to H-ZSM-5 and converted into olefins and aromatics.
- Selective conversion of CO2 and H2 into aromatics,
Youming Ni, Zhiyang Chen, Yi Fu, Yong Liu, Wenliang Zhu, Zhongmin Liu,
Nat. Commun. 2018.
https://doi.org/10.1038/s41467-018-05880-4