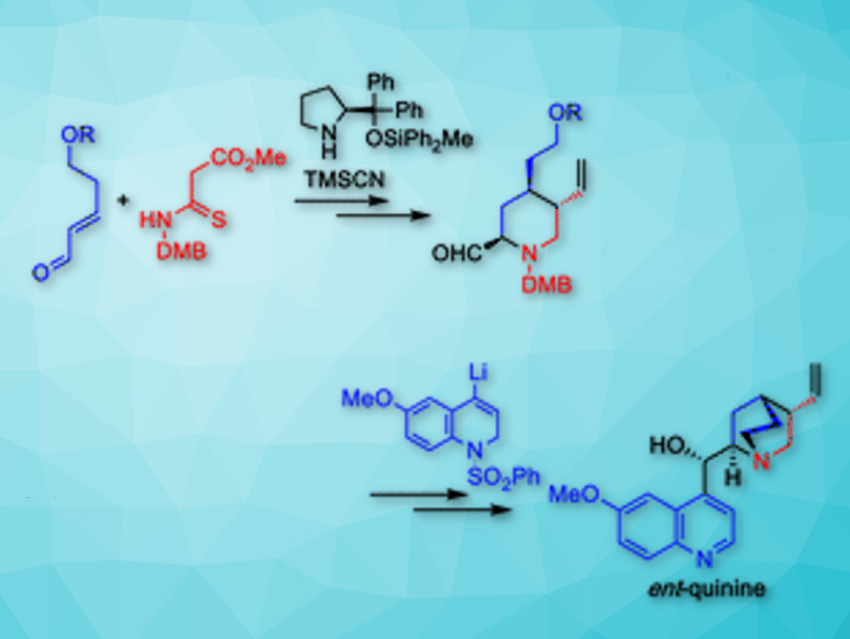
Hayato Ishikawa and colleagues, Kumamoto University, Japan, have reported a 15-step enantioselective total synthesis of the unnatural (+)-quinine and (−)-9-epi-quinine enantiomers. They are important organocatalysts. The protocol provided both (+)-quinine and (−)-9-epi-quinine, each with a 16 % overall yield.
The synthesis started with the enantioselective construction of chiral 4-alkyl piperidine-2-ol from a 5-hydroxypentenal derivative and a thiomalonamate by using an asymmetric formal aza [3 + 3] cycloaddition reaction in the presence of 0.5 mol% diphenylprolinol diphenylmethylsilyl ether catalyst (pictured above). Cyanation of the hemiaminal moiety of the crude diastereomeric product mixture was carried out in a Strecker-type cyanation reaction. Therefore, trimethylsilyl cyanide (TMSCN) and BF3·Et2O were added to the reaction mixture and cyano δ-thiolactam was obtained in 90 % yield over two steps as a mixture of three diastereomers.
In the course of synthesis, an imidate group, derived from a cyano group, was incorporated in the strategy for site-selective modification of the C4-alkyl chiral piperidine ring of quinine. A coupling between the quinuclidine precursor and the dihydroquinoline unit was achieved on a gram scale.
According to the researchers, their synthesis also allows several aromatic groups to be introduced to a tetrasubstituted piperidine derivative, a key intermediate. By this, a wide range of new catalysts, that were previously difficult to derive from naturally occurring cinchona alkaloids, can be synthesized. This may lead to the development of new classes of cinchona alkaloid-mimic catalysis.
- Enantioselective total synthesis of the unnatural enantiomer of quinine,
Shinya Shiomi, Remi Misaka, Mayu Kaneko, Hayato Ishikawa,
Chem. Sci. 2019, 10, 9433-9437.
https://doi.org/10.1039/C9SC03879E