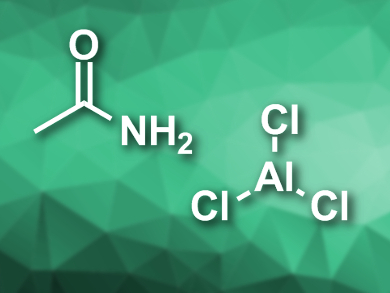
Aluminium-ion batteries (AIBs) use earth-abundant materials, have a high performance, and are generally safer than lithium-ion batteries. These properties could make them suitable for large-scale energy storage. However, currently available AIBs use the expensive ionic liquid 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl), mixed with aluminium chloride, as an electrolyte.
Thomas Nann, Victoria University of Wellington, New Zealand, and colleagues have developed a low-cost alternative electrolyte for AlBs. The team added AlCl3 to anhydrous acetamide under a nitrogen atmosphere to form an eutectic mixture. The resulting pale yellow ionic liquid contains species such as [H3CCONH2–AlCl2]+ and [AlCl4]–.
The prepared ionic liquids were tested as an electrolyte in an electrochemical cell simulating an AlB. The team found charge/discharge profiles which were very similar to those of traditional [EMIm]Cl-based electrolytes. The performance of the acetamide-based electrolyte could be further improved by diluting it with dichloromethane to reduce its viscosity. Acetamide is significantly cheaper than [EMIm]Cl and, thus, this development could reduce the production costs of AIBs significantly.
- Acetamide: a low-cost alternative to alkyl imidazolium chlorides for aluminium-ion batteries,
Nicolo Canever, Nicolas Bertrand, Thomas Nann,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc04468f