There is a wide variety of polyantimonides ([Sbx]n– anions) and transition metal derivatives (e.g., [MSb8]n–). They feature cyclic structures of 3, 4, 5, 7, or 8 Sb atoms. “Naked” Sb6 units or their transition metal derivatives, however, had not been reported so far.
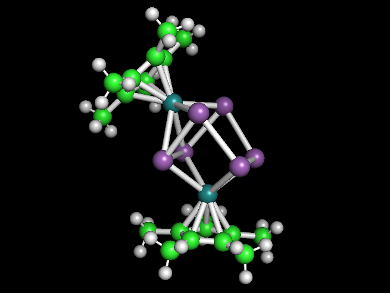
Bryan Eichhorn and colleagues, University of Maryland, College Park, USA, have synthesized the first ruthenium polyantimonide, ([Sb6(RuCp*)2]2– (pictured, Sb in violet, Ru in dark green, Cp* = pentamethylcyclopentadiene). The ion also features the first structurally characterized boat-like Sb6 ring.
The synthesis was performed in a toluene solution using a highly reduced K3Sb intermetallic phase as a [Sbx]n– Zintl ion precursor, [Ru(COD)Cp*Cl] (COD = 1,5-cyclooctadiene) as a Cp*Ru(II) precursor, and [2.2.2]cryptand (2,2,2-crypt) as a ligand for the potassium cations. The reaction gave [K([2.2.2]crypt)]2[Sb6(RuCp*)2] • 2 toluene as dark red crystals.
The product was characterized using single crystal X-ray diffraction, 1H and 13C NMR spectroscopy; energy-dispersive X-ray analysis (EDX), and laser desorption ionization time-of-flight mass spectrometry (LDI-TOF MS). The team found a boat-like Sb6 structure, coordinated to two [RuCp*] fragments. The RuCp* groups are inequivalent in the solid state structure, but are dynamically exchanged in solution, making them equivalent in the NMR spectra. This is consistent with known dynamic exchange processes in related Zintl ions.
- The cyclo-Sb6 Ring in the [Sb6(RuCp*)2]2– Ion,
Yi Wang, Peter Zavalij, Bryan W Eichhorn,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc06542j