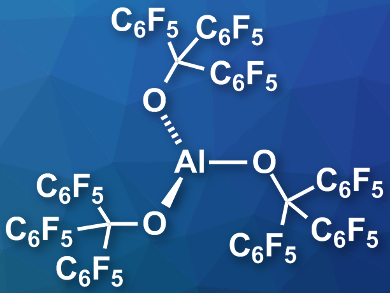
Lewis superacids are defined as compounds with a higher fluoride ion affinity (FIA) than molecular SbF5. They are, for example, used to generate weakly coordinating anions. A commonly used Lewis superacid is Al(OC(CF3)3)3, which, however, has a limited thermal stability and has not been isolated. It is usually prepared in situ.
Julius F. Kögel, University of Bremen, Germany, Alexey Y. Timoshkin, St. Petersburg State University, Russia, and colleagues have prepared the thermally stable, isolable Lewis superacid Al(OC(C6F5)3)3 (pictured). The team reacted triethylaluminum with the alcohol (C6F5)3COH, which gave the desired product in a yield of 66 %. The product is stable up to 180°C and can be stored for long periods under an argon atmosphere at room temperature.
The researchers performed competition experiments for fluoride ions with [SbF6]– and found evidence of their product’s superacidic character and its high FIA. The team also prepared the acid’s corresponding weakly coordinating anions [FAl(OC(C6F5)3)3]– and [ClAl(OC(C6F5)3)3]–, whose salts could be useful, e.g., as oxidants, in salt metathesis reactions, or in hydride or alkyl elimination reactions.
- Al(OCArF3)3 – A Thermally Stable Lewis Superacid,
Julius F. Kögel, Alexey Y. Timoshkin, Artem Schröder, Enno Lork, Jens Beckmann,
Chem. Sci. 2018.
https://doi.org/10.1039/c8sc02981d