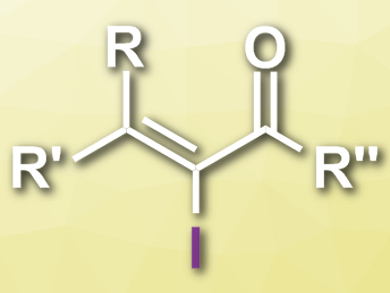
α-Iodoenones (pictured) are useful intermediates in organic synthesis. They can serve as substrates for C–C cross-coupling reactions, leading to α-carbon-substituted enone derivatives. However, most syntheses of α-iodoenones require metal catalysts.
Alfredo Ballesteros and colleagues, University of Oviedo, Spain, have developed a metal-free synthesis of α-iodoenones from propargylic esters. The team used propargylic tosylate or acetate esters as substrates and bis(pyridine) iodonium tetrafluoroborate (Barluenga’s reagent) as a iodinating agent that also promotes a rearrangement of the propargylic esters. The reaction proceeds at 0 °C within 0.5 h and the desired α-iodoenones are obtained in high yields.
The proposed reaction mechanism involves the activation of the carbon–carbon triple bond by a iodonium cation, followed by a nucleophilic attack of the ester group’s oxygen atom on the alkyne, which initiates the rearrangement. The approach can be used for the synthesis of β-unsubstituted, β-substituted, and β,β-disubstituted α-iodoenones.
- Direct Synthesis of α-Iodoenones by IPy2BF4-Promoted Rearrangement of Propargylic Esters,
Tatiana Suárez-Rodríguez, Ángel L. Suárez-Sobrino, Alfredo Ballesteros,
J. Org. Chem. 2018.
https://doi.org/10.1021/acs.joc.8b01746